Abstract
Background: FLT3-ITD mutation in acute myeloid leukemia (AML) is associated with early relapse and poor survival. Quizartinib inhibits FLT3 kinase activity potently and selectively. In phase I and II studies, the composite response rate was approximately 50% among pts with FLT3-ITD. There is in-vitro synergy between quizartinib and 5-azacitidine (AZA) or low dose cytarabine (LDAC). Adding quizartinib to AZA or LDAC may improve the overall response rate (ORR) and duration expected from the use of either agent alone.
The primary objective of phase I is to determine dose limiting toxicity and maximally tolerated dose of combination of quizartinib with either AZA or LDAC; for phase II to determine the clinical activity of both combinations.
Methods: In phase I, pts with relapsed/refractory high-risk myelodysplastic syndrome (MDS), chronic myelomonocytic leukemia (CMML) or AML were eligible irrespective of FLT3 mutation and salvage status. Phase II enrolled pts age >60 years (Y) with untreated MDS/CMML/AML or any age receiving first salvage treatment for AML with FLT3-ITD were eligible. Other requisites: performance status ≤2, adequate organ function and normal electrolytes (potassium, calcium and magnesium). Exclusions include: QTcF>450 msec, administration of drugs that prolong QT/QTc or strong CYP3A4 inhibitors or inducers.
Treatment cycle is 28 days and comprises AZA 75 mg/m2 subcutaneously (SQ) or intravenously for 7 days per cycle, or cytarabine 20 mg SQ twice daily for 10 days per cycle along with quizartinib at 2 planned dose levels: 60 mg (dose level 1) or 90 mg orally daily (dose level 2), uninterrupted. Pts are assigned to AZA or LDAC arm by physician choice.
Results: Sixty-one (Phase I=12, phase II=49) pts have been enrolled: 38 to AZA arm and 23 to LDAC arm, and 59 are evaluable for response (2 too early). Median age is 68 Y (range, 23-84), 27 (44%) are female. Cytogenetics are diploid=25, +8=5, monosomy 7=3, miscellaneous=20, 11q=2, t(8;21) = 1 and unknown=3. Median number of prior therapies is 1 (range, 0-7); 8 pts had received prior FLT3 inhibitor: sorafenib (6), crenolanib (1), quizartinib (1). For both combinations quizartinib 60 mg daily was identified as the recommended phase II dose.
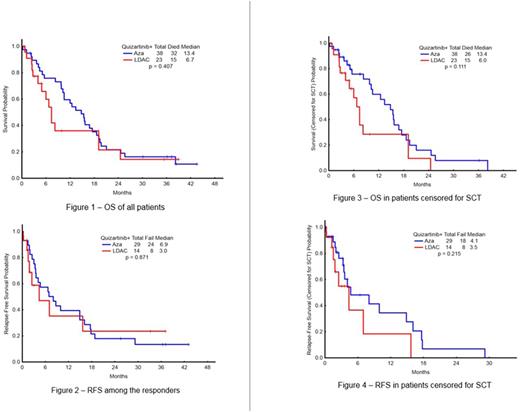
Forty-three pts [14 in LDAC arm (67%) and 29 in AZA arm (76%)] of the 59 evaluable have responded with ORR 73 % (CR=10, CRp=6, CRi=20, PR=2); 5/43 (12%) pts are MRD negative. Twelve pts were previously untreated, and 11 responded (ORR 92%) (CR-6, CRi-1, CRn-1, CRp-2, HI-P-1). The median overall survival (OS) for previously untreated was 18.6 mo (range, 3.91-43.5). ORR among the 47 previously treated was 68% (CR-4, CRi-19, CRp-4, HI-2, HI-E-1, PR-2), and the median OS 11.25 mo (range, 1.15 - 38.93). ORR is 75% among pts with FLT3-ITD mutation (N=55), and 5 (9%) had no MRD detectable (4 AZA, 1 LDAC). Four of five pts (80%) with prior FLT3 inhibitor exposure responded. Median time to response is 65 days (range, 6-289) in AZA arm and 61 (range, 20-256) in LDAC arm. At a median follow-up of 20 mo (range, 0.5-43.5), 11 of the 43 responders remain in CR: 6 had SCT, 4 are continuing study therapy and 1 discontinued due to insurance issues. 29/43 pts failed to respond. Three pts died in CR: due to intra cerebral hemorrhage, post-transplant, and unknown cause, respectively. The median OS was: 20 mo for the total study group: 13.4 mo in AZA arm and 6.7 mo in LDAC arm (p=0.407) (Figure 1); median RFS was 6.9 mo in AZA arm and 3 mo in LDAC arm (p= 0.871) (Figure 2). The median OS for the pts censored for SCT was 13.4 mo in AZA arm and 6.0 mo in LDAC (p=0.111) (Figure 3). RFS for the pts censored for SCT was 4.1 mo in AZA arm and 3.5 mo in LDAC arm (p=0.215) (Figure 4). Fourteen (33%) pts have maintained response for more than 1 Y. Grade 3/4 toxicities irrespective of attribution included hypokalemia (19), hyperkalemia (2), hypotension (9), hypophosphatemia (9), hyponatremia (9), hypocalcemia (5), hyperbilirubinemia (7), elevated ALT (8), elevated AST (4), abdominal pain (3), intra cerebral hemorrhage (2), hypernatremia (2), hypermagnesemia (4), diarrhea (8), dehydration (2), respiratory failure (6), hyperglycemia (1), QTcF prolongation (3), sinus tachycardia (1), atrial fibrillation (4), pericardial effusion (1).
Conclusion: Combination of quizartinib and AZA or LDAC is highly active among AML pts with FLT3-ITD mutation. ORR appear higher than expected with either agent alone. Clinically significant QTcF prolongation is infrequent. Accrual to the study continues.
Kantarjian: Novartis: Research Funding; Delta-Fly Pharma: Research Funding; ARIAD: Research Funding; Bristol-Meyers Squibb: Research Funding; Amgen: Research Funding; Pfizer: Research Funding. Daver: Karyopharm: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy; Pfizer Inc.: Consultancy, Research Funding; Otsuka America Pharmaceutical, Inc.: Consultancy; Bristol-Myers Squibb Company: Consultancy, Research Funding; Immunogen: Research Funding; Jazz: Consultancy; Incyte Corporation: Honoraria, Research Funding; Daiichi-Sankyo: Research Funding; Sunesis Pharmaceuticals, Inc.: Consultancy, Research Funding; Kiromic: Research Funding. DiNardo: Agios: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Celgene: Honoraria, Research Funding. Jain: Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Verastem: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Research Funding; Incyte: Research Funding. Pemmaraju: roche diagnostics: Consultancy, Honoraria; LFB: Consultancy, Honoraria; cellectis: Research Funding; abbvie: Research Funding; novartis: Consultancy, Honoraria, Research Funding; Incyte Corporation: Consultancy, Honoraria; stemline: Consultancy, Honoraria, Research Funding; affymetrix: Research Funding. Jabbour: Bristol-Myers Squibb: Consultancy. Wierda: Sanofi: Consultancy, Honoraria; Kite: Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Acerta: Research Funding; Genentech/Roche: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria, Research Funding; The University of Texas MD Anderson Cancer Center: Employment; Janssen: Research Funding; Karyopharm: Research Funding; Emergent: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria; Genzyme: Consultancy, Honoraria; GSK/Novartis: Consultancy, Honoraria, Research Funding; Juno: Research Funding. Cortes: Teva: Research Funding; Pfizer: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; Sun Pharma: Research Funding; ImmunoGen: Consultancy, Research Funding; ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal